Preclinical
Our Preclinical Expertise
- We support the entire development process, from early-stage R&D through non-GLP proof-of-concept and feasibility studies, into GLP regulatory studies, and ultimately facilitating clinical advancements.
- We utilize both small and large animal models, ensuring that every study is designed in collaboration with the sponsor to tailor models to specific product applications, seamlessly linking in vitro assay development to surgical study endpoints.
- Our expert regulatory guidance and consulting services provide comprehensive support at every stage of development.
Our Facility
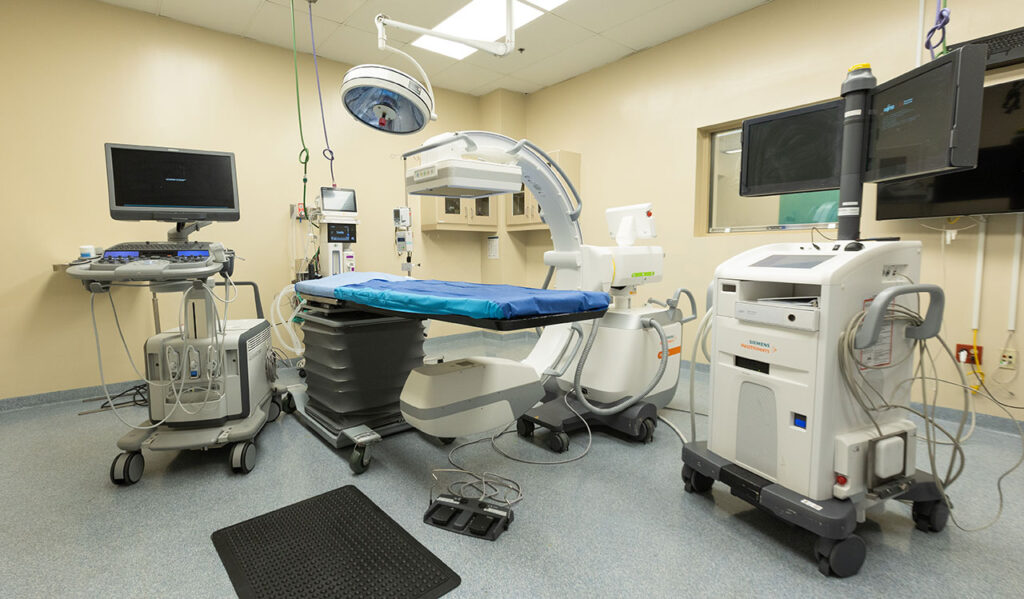
- Our 30,000+ sq. ft. facility consists of procedure suites with attached conference rooms, livestream video capabilities, animal rooms, and dedicated storage closets for clients.
- Our Catheterization (cath) lab features a Siemens Artis Zee fixed, flat detector C-arm while our two alternate operating room suites have mobile C-arm capabilities.
- Element Research Group has a fully equipped necropsy suite and full-time prosector. In addition to full-service necropsy capabilities, we coordinate histological tissue processing. Capabilities include plastic and paraffin embedding and sectioning, as well as common and specialized stains. Pathology services are also coordinated with Board-Certified Veterinary Pathologists.
- Our laboratory support offerings include in vivo blood loop testing, general laboratory space, clinical pathology, -80 degree storage, liquid nitrogen, and more.
Our Equipment
- Fluoroscopy (Siemens Artis Zee Digital Cath Lab)
- Siemens Mobile C-arm
- 3D Rotational Fluoroscopy with Siemens DynaCT
- Intracardiac (ICE) and Duplex Ultrasound (Siemens Acuson SC2000, GE Vivid S70N)
- AD Instruments PowerLab Digital Acquisition System
- DSI-PhysioTel Digital Equipment
- Mindray Veta5 Anesthesia Machines and Animal Monitoring Equipment
- Steam and ethylene oxide sterilization capabilities
- Cardiopulmonary Bypass Pump System
- Cardiopulmonary Bypass & Extracorporeal Membrane Oxygenation (ECMO)
- Intravascular Ultrasound (IVUS) Systems
- Endoscopic and Laparoscopic Tower